Tough new measures aimed at stopping unqualified individuals from carrying out cosmetic procedures have been unveiled by the government.
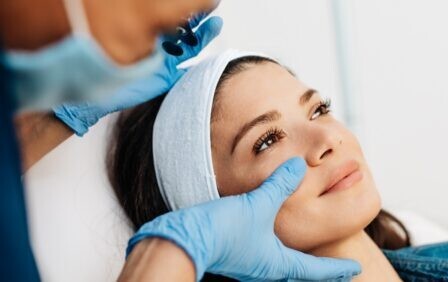
Under the proposals, only suitably qualified healthcare professionals operating from premises registered with the Care Quality Commission (CQC) will be allowed to perform high-risk treatments such as non-surgical Brazilian butt lifts. The Pharmacist has asked the government what the measures mean for pharmacists working in pharmacies, which are regulated by the General Pharmaceutical Council.
These procedures, often marketed as ‘non-invasive’, have been linked to serious complications, permanent disfigurement and even death when carried out by untrained individuals in unregulated settings, according to the Department of Health and Social Care (DHSC).
The plans form part of a broader push to tackle what health minister Karin Smyth described as a ‘Wild West’ of rogue practitioners and unsafe environments, including pop-up clinics, hotels and private homes.
Related Article: Optimising heart failure reviews: practical tips for primary care pharmacists
‘There are countless horror stories of cosmetic cowboys causing serious, catastrophic damage,’ said Ms Smyth.
Alongside stricter controls on high-risk treatments, a new licensing scheme will also apply to lower-risk cosmetic procedures, including Botox and dermal fillers.
Practitioners will be required to meet strict standards around training, hygiene, insurance and premises, with enforcement powers granted to local authorities.
Breaches involving the highest-risk procedures will be subject to CQC enforcement and financial penalties.
Ministers also plan to introduce a ban on under-18s accessing high-risk procedures unless explicitly approved by a healthcare professional.
The age restriction is intended to protect children and teenagers from dangerous beauty trends circulating on social media. Concerns have also been voiced over the online availability of weight-loss treatments.
Related Article: Once-weekly estradiol patches to be discontinued
The announcement by the DHSC follows a public consultation on the licensing of non-surgical cosmetic procedures in 2023, which attracted nearly 12,000 responses.
A further consultation, expected early next year, will seek views on the specific treatments to be covered under the new regime, with priority given to procedures involving the breasts and genitals.
In addition to improving public safety, the measures aim to save the NHS significant time and money by reducing the number of corrective treatments required after botched procedures.
Recent investigations by the UK Health Security Agency and the Medicines and Healthcare products Regulatory Agency have highlighted the risks posed by unlicensed or misused products, including botulinum toxin.
Industry figures have welcomed the government proposals as a long-overdue step toward formalising standards in the rapidly growing aesthetics sector.
Related Article: RSV vaccine drive for pregnant women as pharmacy role expands
Millie Kendall, chief executive of the British Beauty Council, said: ‘Any measures that increase protection for the general public and professionalise the industry will help instil confidence, as well as helping to prevent the normalisation of horror stories that have become synonymous with our sector.’
Professor David Sines, executive chair of the Joint Council for Cosmetic Practitioners, added: ‘The introduction of standards to ensure that patients are safeguarded and protected from harm… has become imperative. These proposals have our full support.’

Have your say
Please add your comment in the box below. You can include links, but HTML is not permitted. Please note that comments are not moderated before publication and the views expressed are those of the user and do not reflect the views of The Pharmacist. Remember that submission of comments is governed by our Terms and Conditions. You can also read our full guidelines on article comments here – but please be aware that you are legally liable for any libellous or offensive comments that you make. If you have a complaint about a comment or are concerned that a comment breaches our terms and conditions, please use the ‘Report this comment’ function to alert our web team.